Chelsea M. Berns, Ph.D.
Ph.D. in Ecology and Evolutionary Biology
Contribution of a chapter in the book Sexual Dimorphism
The evolution of sexual dimorphism: Understanding mechanisms of sexual shape differences
Understanding the origin of biodiversity has been a major focus in evolutionary and ecological biology for well over a century, and several patterns and mechanisms have been proposed to explain this diversity. Particularly intriguing is the pattern of sexual dimorphism, in which males and females of the same species differ in some way. Sexual dimorphism is a trait that is seen throughout the animal kingdom and is exhibited in a myriad of ways, such as differences in coloration, behavior, vocalizations, ornamentation and size (see Fig. 1). Its consequences for the ecology and evolution of organisms are often profound, and a number of mechanisms have been proposed to explain its variable distribution among taxa.
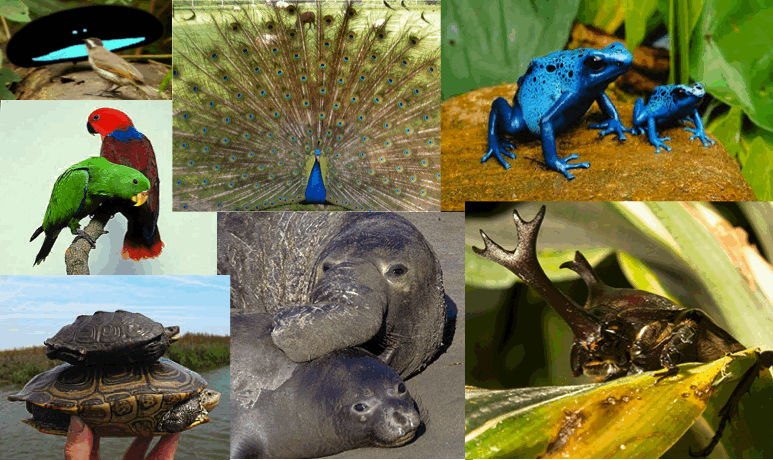
Fig. 1 Instances of sexual dimorphism in coloration, size, mating diaplay behavior, and ornamentation. (See photo credits at bottom)
Darwin suggested that an advantage in mate choice or competition for mates may be mechanisms that underlie sexual dimorphism and indeed, in several taxa competition for mates has been shown to be the major factor impacting sexual dimorphism. For example, many researchers have argued that competition for mates is at the very heart of sexual selection, because these rivalries greatly influence mating and fertilization success. It has also been proposed that differences in reproductive behavior between the sexes are a driver in the evolution of sexual dimorphism in many species. Other hypotheses have been proposed to explain patterns of sexual dimorphism, such as sex-specific divergent selection in response to environmental gradients by each sex and differing trade-offs between traits related to fitness.
When processes of sexual size dimorphism occur in closely related species, distinct patterns of among species can result. One such pattern is called ‘Rensch’s Rule’ in which sexual size dimorphism increases with body size in species where males are of larger size than females, and decreases in those species where females are the larger sex (see Fig. 2). Another such pattern is the ‘adaptive canalization’ hypothesis, stating that if the larger sex is under strong selection for a large body, individuals with sub-optimal body sizes will have lower fitness. An additional pattern is that of condition-dependence. Under this hypothesis, if the larger sex is under stronger directional selection for a large size, it will be more affected by different environmental factors as compared to the smaller sex thus, sexual size dimorphism should change with changing environments.
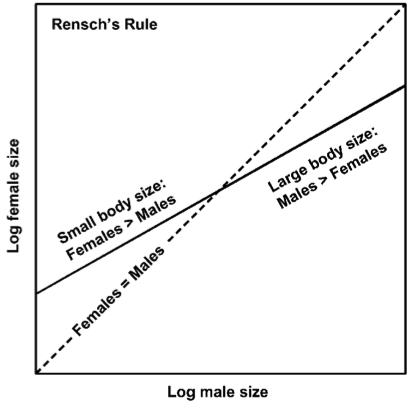
Fig. 2 Rensch's Rule, from Colwell 2000
In addition to sexual size dimorphism, males and females often differ widely in shape. Curiously, although shape can contribute meaningfully to various functions such as feeding, mating, parental care, and other life history characteristics, sexual shape dimorphism has historically received considerably less attention than sexual size differences. Examining the size and shape of traits together is much more complete in the quantification of sexual dimorphism, as they are not necessarily independent. Shape allows a deeper understanding of mechanisms underlying sexual dimorphism because different parts of the body can serve multiple functions.
Recently, sexual shape dimorphism has been identified in numerous taxa, including dipterans, fish, turtles, hummingbirds and lizards, to name a few. In those cases that have been studied, both natural and sexual selection have contributed to the evolution of shape differences between the sexes. For instance, much research on sexual shape dimorphism has focused on ontogenetics. For example, in Podarcis lizards it was found that, while the immatures were more similar in head size, they differed significantly in head shape. Another study found sexual shape dimorphism in the hatchlings of the Chelydra serpentina turtle which appears to be consistent to that observed in adults, indicating that sexual shape dimorphism during this ontogeny may simply be a precursor to the adult dimorphism. Even another study has revealed ontogenetic trajectories for sexual shape dimorphism by demonstrating that in Hemideina crassidens, sexual selection has driven differences in head shape with a common ontogenetic trajectory for both sexes.
Other studies are beginning to examine convergent parallelism and conserved genetic factors as well, finding that Telostylinus angusticollis flies have congruent patterns of shape variation interspecifically which do not seem to be due to common life-history requirements. These flies are in different environments and niches, thus selection on body shape may differ as well. Finally, a study on parallel evolution of species of guppies (Poecilia reticulate) examined the extent to which environmental gradients underlie sexual dimorphism and even went further to examine the effect of predation on this dimorphism. Results indicate that populations experiencing high predation had males with smaller heads and deeper caudal peduncles. Open canopy sites resulted in selection for females with smaller heads and distended abdomens, whereas both sexes in high flow sites had small heads and deeper caudal peduncles.
Although these and other studies are currently underway, many questions about sexual shape dimorphism still remain. For instance, how frequently is sexual shape dimorphism exhibited and how is this related to ontogenetic, biomechanical and phylogenetic influences? Does sexual shape dimorphism follow well-known patterns of sexual size dimorphism, such as Rensch’s Rule? Perhaps variation in morphology is correlated with disruptive selection, which then may impact patterns of sexual shape dimorphism and perhaps is a mechanism underlying speciation. If this is the case, then it is expected that as species numbers increase, sexual shape dimorphism decreases because as species richness increases, sexual shape dimorphism becomes converted to interspecific differentiation rather than intraspecific variation.
With the advent of new phylogenetic techniques, we can begin further examining the details of the evolution of sexual shape dimorphism. What role does sexual shape dimorphism play in microevolutionary patterns and what are the mechanisms underlying these patterns? What might result when scaling these patterns from micro to macroevolution? Questions such as these could be tested by examining patterns of dimorphism in two closely related species, then scaling up to family, genera and so forth. It is now also possible to ask if rates of evolution differ between species and if these rates differ more broadly between different sexually dimorphic traits. What effect does habitat and environmental gradients play in assessing rates and patterns of sexual shape dimorphism evolution? By examining the possible correlation between sexual shape dimorphism and habitat variables in a phylogenetic manner, it is possible to quantify hypotheses such as these.
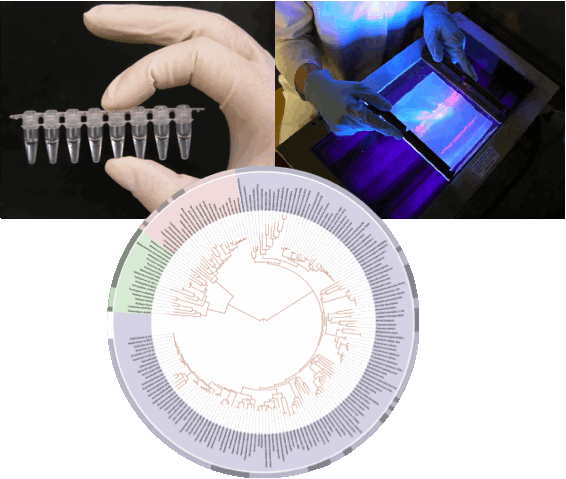
Fig. 3 Phylogenetic techniques enabling the Tree Of Life project
For centuries, biologists have asked "why are there so many species, and why do we see such magnificent biological diversity?" Natural philosophers such as Aristotle and Linnaeus have attempted to organize and understand observable patterns of biological diversity, but it wasn't until Darwin that a theory of mechanisms underlying sexual dimorphism came into focus. While many studies continue to examine sexual size dimorphism, sexual shape dimorphism has received comparatively less attention. Tools now exist that can quantify detailed shape variation, and new phylogenetic techniques allow comparative analyses. Armed with the ability to study these hypotheses, we will be better able to reveal a richer description of the mechanisms and patterns of the evolution of sexual dimorphism.
The full book chapter is scheduled to come out December 2012, so stay tuned!
Photo credits: Eclectus:http://www.buffaloparrot.com/eclectus.JPG
Birds of paradise: http://lsnhs.leesummit.k12.mo.us/webclasseslsn/itw11-12/dw/23a/katherinedw/images/bop-dancenfemale.jpgTurtle: http://researchmagazine.uga.edu/aa/fall2008/images/articles/terrapinMale-Female.jpg
Peacock: http://www.creativitypost.com/images/uploads/activism/peacock2.jpg
Frog: http://thestablewomangazette.net/wp-content/uploads/2012/10/poisonous-frog.jpg
Beetle: http://www.aaas.org/news/releases/2012/images/0726sp_plumage_beetle.jpg
Seal: http://animaldoor.blogspot.com/2011/09/elephant-seal.html
PCR: http://upload.wikimedia.org/wikipedia/commons/thumb/8/81/PCR_tubes.png/300px-PCR_tubes.png
Gel: http://www.coextra.eu/images/image250
Tree of life: http://upload.wikimedia.org/wikipedia/commons/thumb/1/11/Tree_of_life_SVG.svg/350px-Tree_of_life_SVG.svg.png