Chelsea M. Berns, Ph.D.
Ph.D. in Ecology and Evolutionary Biology
Berns, C.M. & D.C. Adams. 2012. Becoming different but staying alike: patterns of sexual size and shape dimorphism in hummingbirds. Evolutionary Biology (40: 246-260).
Understanding the origin of phenotypic diversity is a major focus of evolutionary research, and patterns of sexual dimorphism represent a particularly intriguing component of this diversity. A number of mechanisms have been proposed to explain the evolution of sexual dimorphism. For example, sexual selection can generate sex-specific differences as the sexes evolve in distinct directions that maximize their own reproductive success. Alternatively, ecological mechanisms such as competition for resources, may exert distinct selective forces on the sexes, resulting in the evolution of sexual dimorphism. A third possible mechanism that may enhance sexual dimorphism in some species is the influence of sex-specific divergence in response to environmental gradients, where males and females exhibit differential responses to the same environmental selective pressures.
Hummingbirds (family Trochilidae) offer a unique opportunity to study patterns of sexual dimorphism and elucidate the underlying mechanisms responsible for these patterns, as they continue to be a model taxon due to their dimorphic plumage, behavior, body size and ornamentation. Hummingbirds also exhibit sexual dimorphism in bill morphology, a trait under strong selection pressures due to its role in foraging, and differential foraging among as well as within species is thought to be a major cause of diversification in trophic structures.
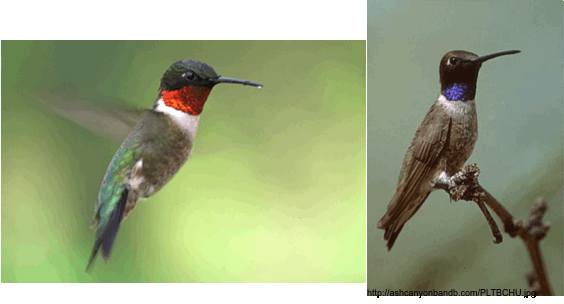
Fig. 1 Left: Ruby-throated Hummingbird (Archilochus colubris), Right: Black-chinned Hummingbird (A. alexandri).
Recently, it was observed that two sister species within the Mellisugini clade, Archilochus alexandri and A. colubris, differ in their patterns of dimorphism in bill morphology (see Fig. 1; Berns and Adams 2010), where both species display significant bill size dimorphism, but only A. colubris exhibits bill sexual shape dimorphism. These results were surprising, as prior work on Trochilidae in other clades suggested that shape dimorphism in bill curvature is common. Given these findings, I asked the following questions:
is the presence and pattern of sexual shape dimorphism in the Mellisugini clade concordant with those found across all Trochilidae?
Is sexual dimorphism in bill size common across species in the Mellisugini clade?
I addressed these questions in a phylogenetic context using both linear measurements and landmark-based geometric morphometric techniques to quantify sexual size and shape dimorphism in the bill morphology in 32 of the 35 Mellisugini species (McGuire et al. 2009).
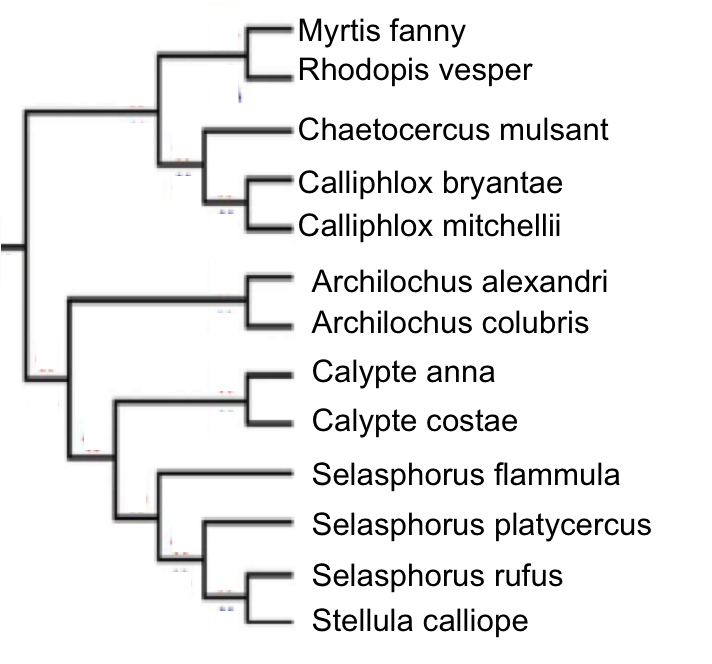
Fig. 2 Phylogeny for a subset of species in the Mellisugini hummingbird clade. Adapted from McGuire et al., 2007.
From the Mellisugini clade, I quantified patterns of phenotypic evolution by examining both sexual size and shape dimorphism in the bills of 30 species. In order to quantify bill morphology, I collected data using landmark-based geometric morphometrics. Briefly, I took lateral-view images of the bills of 1,347 museum hummingbird specimens. I then used landmark-based geometric morphometrics (see Fig. 3) to obtain shape information and performed statistical analyses to quantify bill morphology both between and within species (see Berns and Adams, 2010, 2012 for details).
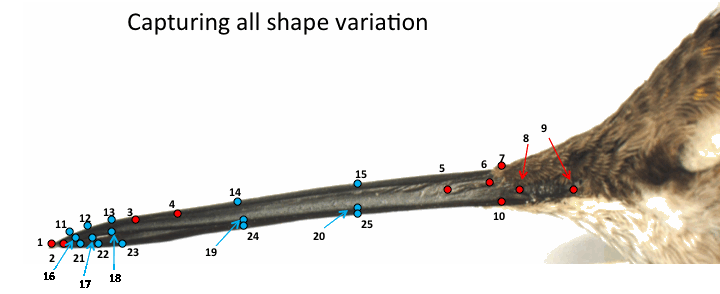
Fig. 3 Landmarks in images of hummingbird bills to capture shape variation. Red landmarks represent biologically homologous points, blue indicates semi-landmarks.
Second, I performed a family-wide analysis of bill size dimorphism, using the bill size measurements of the 32 species in the Mellisugini clade as above, as well measurements of an additional 187 species obtained from literature (Temeles et al. 2010; Colwell 2000; Bleiweiss 1999). In total, this analysis included 219 of 338 hummingbird species, or 65% of the current diversity of the group. From these measurements, the average male and female bill sizes were obtained and used for further analyses.
From the size analyses, I identified considerable variation in the degree of size dimorphism exhibited among species in this group, with a 27-fold difference in the degree of sexual size dimorphism between the species with the least size dimorphism and the most dimorphic species. When magnitudes of sexual size dimorphism were examined across the entire hummingbird family, I found no difference in the degree of size dimorphism exhibited among clades within Trochilidae.
From the shape analyses, I found that only 3 species of the 32 species examined exhibit sexual shape dimorphism. When patterns of sexual shape dimorphism were compared among these three species, one exhibited a significantly greater degree of shape dimorphism whereas the two others did not differ in the amount of shape dimorphism displayed.
The presence and magnitudes of sexual dimorphism observed here may be the consequence of differential selective forces that result from competition for ecological resources. For example, in species where the males are the dominant sex and defend territories, females tend to have longer bills. In lekking mating systems however, males no longer hold territories. In this case, the male bill tends to be longer to allow them to feed from a wider variety of flowers due to competitive forces, whereas females feed from small patches outside the lekking grounds and have smaller bills better suited to feeding in small patches. Another possible explanation for these patterns is that hummingbirds in species-poor environments may have increased intraspecific competition, as the lack of interspecific competitors would allow the sexes to utilize distinct niches that would otherwise be occupied by congenerics.
Interestingly, the available data suggest the hypothesis that both bill size dimorphism and bill shape dimorphism arose early in the diversification of Trochilidae, and that the lack of sexual shape dimorphism presently displayed in the Mellisugini lineage is a derived trait (see Fig. 4). Taken together, the evolutionary changes in patterns of sexual size and shape dimorphism observed in Mellisugini suggest that the trends of sexual dimorphism in the Trochilidae are far more varied than was previously believed.
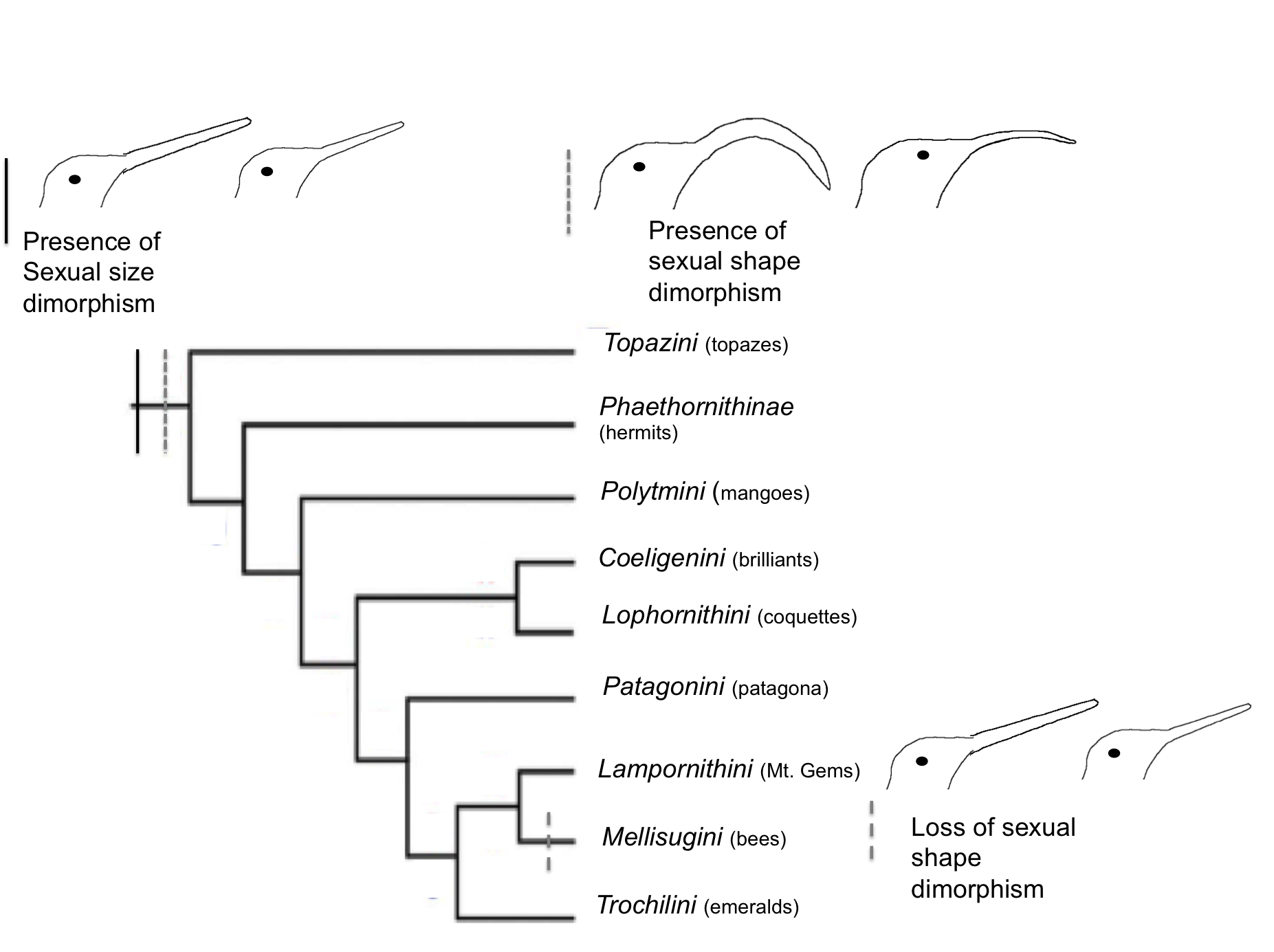
Fig. 4 Current phylogeny of Trochilidae. Representation of magnitude of sexual size dimorphism and sexual shape dimorphism. Adapted from McGuire et al., 2007.
For details, see:
Berns, C.M. & D.C. Adams. 2012. Becoming different but staying alike: patterns of sexual size and shape dimorphism in hummingbirds. Evolutionary Biology (accepted).
Berns, C.M. & Adams, D.C. Bill shape and sexual shape dimorphism between two species of temperate hummingbirds: Black-chinned Hummingbirds (Archilochus alexandri) and Ruby-throated Hummingbirds (Archilochus colubris). The Auk 127, 626-635 (2010).
McGuire, J.A., Witt, C.C., Altshuler, D. & Remsen, J.V. Phylogenetic systematics and biogeography of hummingbirds: Bayesian and Maximum Likelihood Analyses of partitioned data and selection of an appropriate partitioning strategy. Systematic Biology 56, 837-856 (2007).
McGuire, J., Witt, C., Remsen, J., Dudley, R. & Altshuler, D. A higher-level taxonomy for hummingbirds. Journal of Ornithology 150, 155-165 (2009).