Chelsea M. Berns, Ph.D.
Ph.D. in Ecology and Evolutionary Biology
Why are there so many species and why do we observe such magnificent biological diversity? These longstanding questions have been studied by biologists for over a century, and many hypotheses have been proposed to explain the mechanisms driving this diversity as well as the patterns that we observe. One such hypothesis predicts that adaptive phenotypic divergence promotes the rapid evolution of new species ("the ecological theory of adaptive radiation", sensu Schluter, 2000). This theory posits that the primary factor driving divergence and speciation is the evolution of phenotypic adaptation. Specifically, new species develop the ability to utilize empty niche space due to adaptive morphological divergence and resource specialization.
In lineages that have undergone an adaptive radiation, a number of specific morphological patterns are expected to emerge. As such, there are several explicit and testable predictions that can be made to examine whether a particular lineage exhibits patterns that may signify an adaptive radiation. For example, it has been suggested that morphological variation within subclades will be positively associated with the species richness in a clade. Additionally, some investigators have predicted that the taxa whose morphological diversification arises relatively early in their histories will partition more of their morphological changes among subclades. Further, it is hypothesized that adaptive radiations will exhibit a correlation between species richness and the degree of morphological variation in monophyletic subclades. Such patterns may lead to the association of the rates of species diversification and morphological divergence.
In this study (Adams et al., 2009) we quantified morphological and phylogenetic data using plethodontid salamanders as a model organism in order to test these predictions of an adaptive radiation. We calculated diversification rate for each clade using the number of species and the age of the clades and used the method-of-moments estimator for crown groups to obtain diversification rates (Magallon & Sanderson, 2001). We gathered morphological data from 1573 adult plethodontid specimens from 260 species in 26 genera. From these specimens, we recorded: snout-vent length, tail length, body width, head length, snou-eye distance, forelimb length and hindlimb length (see Fig. 1). Finally, we calculated rates of (i) species diversification, (ii) morphological evolution in body size and (iii) morphological evolution in body shape.
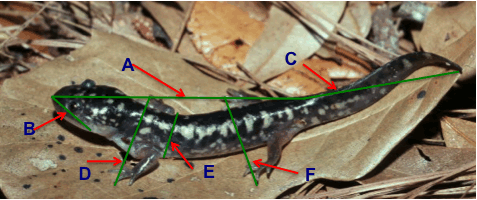
Fig. 1 Standard morphometric variables measured to the nearest 0.1 mm using digital calipers: A) snout–vent length, B) head length (tip of snout to posterior corner of jaw), C) tail length (posterior end of cloaca to outstretched tail tip, D) forelimb length (posterior insertion of forelimb to outstretched tip of longest finger), E) body width (just posterior to forelimb insertion), F) hindlimb length (posterior insertion of hindlimb to outstretched tip of longest toe). Adapted from http://a-z animals.com/media/animals/images/original/tiger_salamander3.jpg
Surprisingly, we found that rates of species diversification and morphological evolution are not significantly correlated. This implies that speciation in this group is not accompanied with great amounts of phenotypic divergence. This may be due to other factors which more heavily influence reproductively isolated populations- for instance, climactic factors. Our results also imply that patterns of phenotypic evolution in body form are similar across the plethodon groups we examined, regardless of rates of species diversification or presence of other sympatric clades.
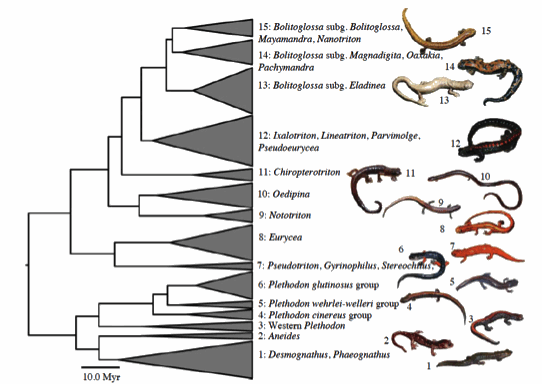
Fig. 2 Branch lengths represent age, width of clades represents the proportion of species currently recognized.
References:
Adams, D.C., Berns, C.M., Kozak, K.H. & Weins, J.J. Are rates of speciation correlated with rates of morphological evolution? Proceedings of the Royal Society of London Series B-Biological Sciences (2009).
Schluter, D. The Ecology of Adaptive Radiation (eds. Harvey, P.H. & May, R.M.) (Oxford University Press, Oxford, England, 2000).
Magellan, S. & Sanderson, M.J. Absolute diversification rates in angiosperm clades. Evolution 55, 1762-1780 (2001).